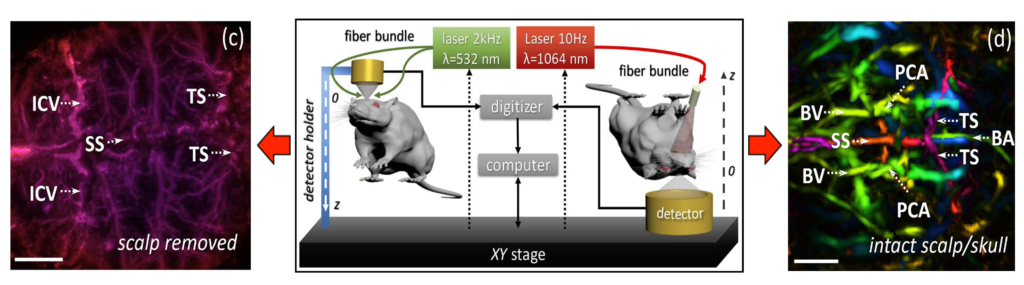
Cerebrovascular imaging of rodents is one of the trending applications of optoacoustics aimed at studying brain activity and pathology. Imaging of deep brain structures is often hindered by sub-optimal arrangement of the light delivery and acoustic detection systems. In our work we revisit the physics behind opto-acoustic signal generation for theoretical evaluation of optimal laser wavelengths to perform cerebrovascular optoacoustic angiography of rodents beyond the penetration barriers imposed by light diffusion in highly scattering and absorbing brain tissues. A comprehensive model based on diffusion approximation was developed to simulate optoacoustic signal generation using optical and acoustic parameters closely mimicking a typical murine brain. The model revealed three characteristic wavelength ranges in the visible and near-infrared spectra optimally suited for imaging cerebral vasculature of different size and depth. The theoretical conclusions are confirmed by numerical simulations while in vivo imaging experiments further validated the ability to accurately resolve brain vasculature at depths ranging between 0.7 and 7 mm.
Subochev P., Smolina E., Sergeeva E., Kirillin M., Orlova A., Kurakina D., Emyanov D., and Razansky D. Toward whole-brain in vivo optoacoustic angiography of rodents: modeling and experimental observations. Biomedical Optics Express 11(3):1477-88, (2020).
Follow the link below to read the article:
